Ans In the third period we are provided that element. Three days after the United States dropped an atomic bomb on Hiroshima a second atomic bomb was dropped on Nagasaki on August 9 a 21-kiloton plutonium device known as Fat Man On the day of the bombing an estimated 263000 were in Nagasaki including 240000 Japanese residents 9000 Japanese soldiers and 400 prisoners of war.
Periodicity Trends Along Period 3 A Level Chemistry Study Mind
It is defined as half of the intemuclear distance between the two adjacent metal ions in the metallic lattice.

. So this means that sometimes atoms with greater atomic mass are smaller in size than. Across a period the atomic radius decreases from left to right. D Metallic Radius.
Exceptions are observed in transition metal elements. This is because Chlorine has a larger. As you go across a period the atomic radius decreases.
Robert and Amelia Krauss. Because the outer electrons are present in the same valence shell during a period and the atomic number increases from left to right across a period the effective nuclear charge increases. Along a period the atomic radii of.
First ionisation energy across period 3. Element is in 3 rd period of the periodic table. This is because the effective positive force of the nucleus also increases drawing in the electrons more tightly.
The 509th Remembered A History of the 509th Composite Group as Told by the Veterans that Dropped the Atomic Bombs on Japan. Return of the Enola Gay. Variation of Atomic radii across a period.
Atomic radii decrease with the increase in the atomic number in a period. As a result electrons become more attracted to the nucleus. This is due to continuous increases in the number of electronic shells or orbit numbers in the structure of atoms of the elements down a group.
Across a period the effective nuclear charge increases. Melting and boiling points across period 3. The atomic radius in the periodic table decreases across the period and increases down the group.
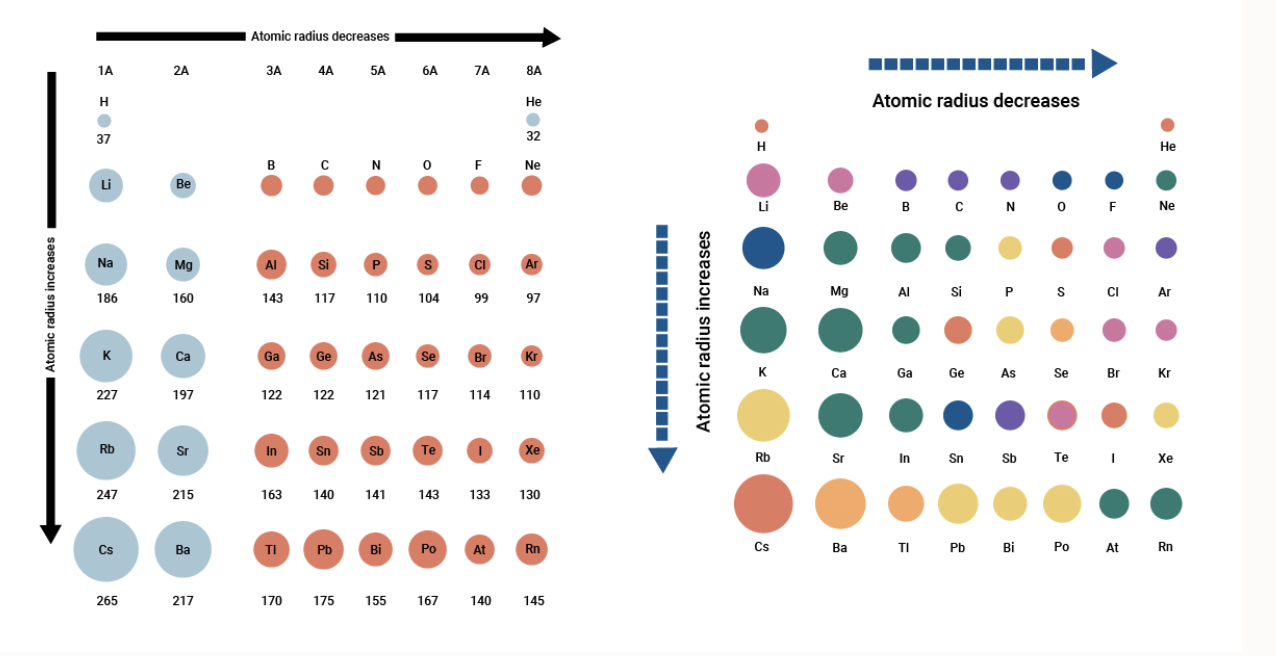
In moving from left to right across the period the nuclear charge increases progressively by one unit but the additional electron goes to the. Within a group Atomic radius increases down the group. The first atomic radius periodic trend is that atomic size decreases as you move left to right across a period.
Atomic Radii Decrease From Left to Right Across a Period. Why does the atomic radius decrease across a period. Within a period of elements each new electron is added to the same shell.
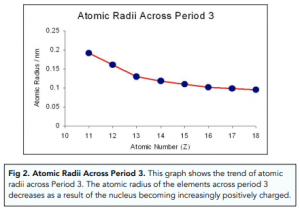
As you go down a group the atomic radius increases. There is a lot going on in this graph so it is often easier to divide it into three sections. Even though the size of the atomic nucleus increases with larger atomic numbers moving across a period the ionic and atomic radius decreases.
When an electron is added a new proton is also added to the nucleus which. A loop of radius r 30 cm is placed parallel to the xy-plane in a uniform magnetic field B. For example Sodium in period 3 has an atomic radius of 186 picometers and chlorine in the same period has an atomic radius of 99 picometers.
The modern table is derived from Mendeleevs periodic table but with. Period of the circular motion of the alpha particles is closest to A 044 μs. Atomic Radius Trend 1.
Element is in 17 th group of the periodic table. The table below gives a brief summary of these sections. The storage rings of SSRF and TPS were operated at 35 and 30 GeV with a maximum electron current of 250 and 500 mA respectively.
The Half-Life of History the Atomic Bomb and Wendover Air Base. The data were collected in fluorescence mode using a seven. The atomic weight of copper is 633 gmol and its density is 8900 kgm3.
The highest principal quantum number n of the element into which the last electron enters is known as the period number. Atomic radius across period 3. From left to right across a period atomic radii generally decreases due.
The periodic table is a tabular arrangement of the chemical elements by increasing atomic number which displays the elements so that one may see trends in their propertiesThe Russian scientist Dmitri Mendeleev is most often credited with inventing the periodic table 1869. Online timeline of the bombing of Nagasaki. For example atomic radii decrease from lithium to fluorine in the second period.
Across a period the number of electron shells remains the same while the number of electrons increases. The measurement from the centre of the nucleus of an atom till the last shell of electrons of any given element is referred to as the atomic radius. Variation of Atomic Radius in the Periodic Table Variation in a Period.
As you move from left to right across an element period row the ionic radius decreases. The graph shows how melting points and boiling points vary across period 3. It can imply both covalent and metallic radius based on whether the.
Atomic radii tend to decrease as time passes.

Periodic Trends Variation In Atomic Radii Of Elements In Different Blocks Chemistry Stack Exchange

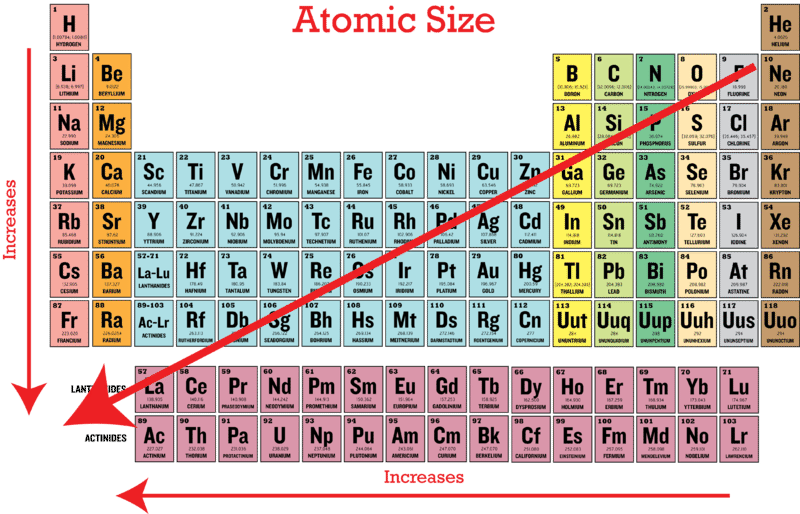
Does Atomic Size Increase Down A Group

A Level Gce Period 3 Element Trends In 1st Ionisation Energy Atomic Radius Pauling Electronegativity Melting Point Boiling Point Electrical Conductivity Density Trends Graphs Plots Discussed Explained Ks5 Revision Notes

Trends In The Periodic Table Chpt 7 1 Atomic Radius Size 2 Ionization Energy 3 Electronegativity The Ionization Energy Periodic Table Covalent Bonding

Periodic Trends In Ionic Radii Chemwiki Ionic Radius Ionization Energy Element Chemistry
How Does Atomic Radius Change As You Move Across The Periodic Table Quora

How To Describe The Trends In The First Ionization Energy Within Groups And Across Periods In The Periodic Table Can You Provide An Example Quora
How Would You Determine Which Element Has A Larger Or Smaller Atomic Radius Quora
Atomic Radius Trend Periodic Table Chemtalk

3 2 Trends In Ionic Radii Sl Youtube
3 2 Periodic Trends Ib Alchemy

Periodic Trends Definition And Properties

Mr Lee Says Atomic Radius Increases As You Go Down A Group Because You Are Adding More Energy Levels Chemistry Lessons Chemistry Education Teaching Chemistry
Periodic Trends In Atomic Size Ck 12 Foundation

The Size Of Atom Decreases Across A Period From Left To Right Description From Streamscience Blogspot Com I Searched For This On Bing Com Images พ นธะเคม